A 30-year legacy in neonatal care
A 30-year legacy in neonatal care
Chiesi’s history in neonatal care highlights a commitment to partnering with healthcare professionals (HCPs) to improve outcomes for infants. Driven by a mission to enhance global neonatal health, we focus on innovative solutions and research to support the best start for every infant.
Our neonatology site is tailored to equip HCPs in the UK and Ireland with the essential information and resources needed to provide the best possible care for their patients.

Explore our treatments
A recommended choice in neonatal care, endorsed by the European Consensus Guidelines (ECG) for treating infants with or at risk of developing RDS or who have surfactant deficiency.1
CUROSURF is indicated for:2
- The treatment, including early rescue, of RDS or hyaline membrane disease in neonates
- Prophylactic use in premature infants requiring intubation for stabilisation at risk from RDS or with evidence of surfactant deficiency
About RDS and the role of CUROSURF in its treatment.
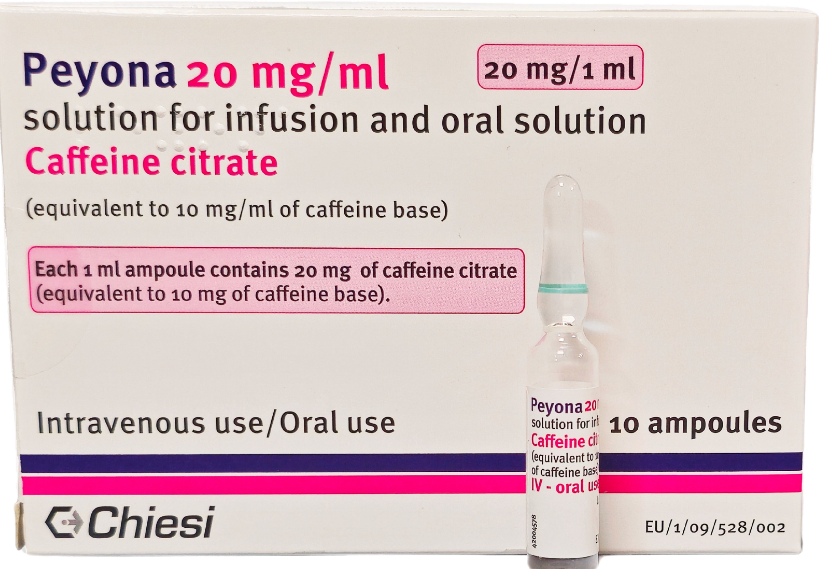
Each 1 ml ampule of Peyona contains 20 mg caffeine citrate (equivalent to 10 mg of caffeine).3
Treatment with caffeine citrate should be initiated under the supervision of a physician experienced in neonatal intensive care. Treatment should be administered only in a neonatal intensive care unit in which adequate facilities are available for patient surveillance and monitoring.3
Peyona is indicated for
- The treatment of primary apnoea of premature neonates
About primary apnoea in newborns and the role of Peyona in its treatment.
Ready to enhance your neonatology skills?
Created in partnership with neonatology experts, our dedicated Medical Education programmes offer a combination of theory and hands-on training to share best practices for the exemplary care of infants — suitable for multiple skill levels including Advanced Neonatal Nurse Practitioners, Senior Registrars, Neonatal Consultants and others.

Explore Chiesi Neonatology
Sustainability
Sustainable choices for a brighter future 
Chiesi is proud to be one of the few pharmaceutical companies that has achieved BCorp certification; joining a global collaborative who are leading the movement for an inclusive, equitable, and regenerative economy.4
We believe in operating through a ‘Shared Value’ approach, by connecting company progress to social and environmental welfare.
Explore our long-term initiatives and tangible steps towards a sustainable future — it may surprise you!
Abbreviations
ECG, European Consensus Guidelines; HCP, healthcare professional; RDS, respiratory distress syndrome; SmPC, Summary of Product Characteristics.
References
- Sweet DG, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology. 2023;120(1):3–23.
- CUROSURF SmPC. Available at https://www.medicines.org.uk/emc/product/6450/smpc. Accessed September 2024.
- Peyona SmPC. Available at https://www.medicines.org.uk/emc/product/4098/smpc. Accessed September 2024.
- BCorporation. Available at https://bcorporation.eu/what-is-a-b-corp/what-does-b-corp-certification-mean/ Accessed September 2024.
IE-NEO-2400145 | December 2024
Adverse event reporting
For the UK: Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Chiesi Limited on 0800 0092329 (UK) or PV.UK@Chiesi.com.
For Ireland: Adverse events should be reported to HPRA Pharmacovigilance – www.hpra.ie. Adverse events should also be reported to Chiesi Limited on 1800 817459 (IE) or PV.UK@Chiesi.com.