CUROSURF by LISA
CUROSURF by LISA
CUROSURF reduces the need for mechanical ventilation and improves oxygenation, regardless of the method of administration.1–6 However, it has become evident in recent decades that invasive mechanical ventilation can cause lung injury, making non-invasive respiratory support an alternative approach for preterm infants.6,7
Less invasive surfactant administration (LISA), also referred to as less invasive surfactant therapy (LIST) or minimally invasive surfactant therapy (MIST), involves delivering surfactant through a thin endotracheal catheter in infants who are breathing on their own and receiving CPAP support.8,9
Multiple clinical studies have shown that LISA is more advantageous than mechanical ventilation as it lowers the risk of BPD, major complications, and in-hospital mortality.9–11
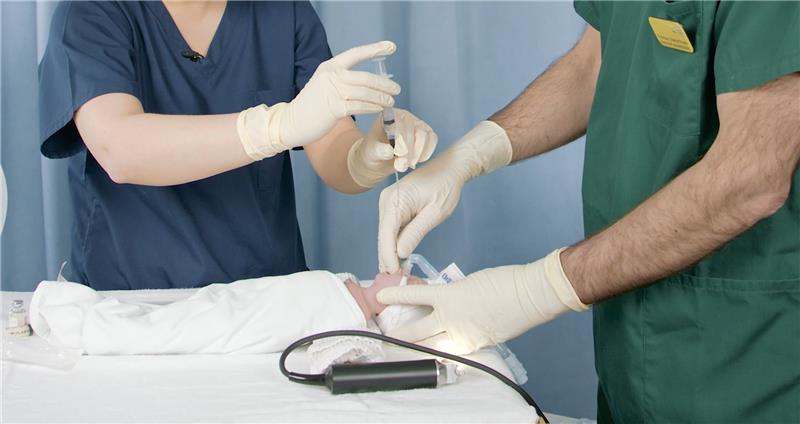
European Consensus Guidelines gives their highest recommendation for surfactant administration using the LISA techniques for spontaneously breathing infants on CPAP (A1)6
BAPM guidelines recommend non-invasive ventilation with early rescue by LISA for the optimal management of infants with RDS, provided clinicians are competent in the required technique12
LISA administration
Learn from an expert neonatologist how LISA uses a thin endotracheal catheter to deliver surfactant to spontaneously breathing infants on CPAP, simplifying the process and enhancing neonatal care with minimal disruption.
Watch our 6 minute video to see how to perform the LISA technique!
Join us in investing in your professional growth and staying at the forefront of a rapidly evolving medical landscape!
LISA reduces the need for mechanical ventilation compared with standard care13
- In a parallel group, randomised controlled trial involving 220 preterm infants born at 26 to 28 weeks’ gestation and weighing less than 1.5 kg, LISA significantly reduced the need for mechanical ventilation compared with standard care (p<0.0001; NNT=3).13
Percentage of infants not intubated/mechanically ventilated after receiving surfactant via standard care or LISA
Adapted from Gopel, et al.13
LISA improves outcomes versus INSURE or delayed ET tube removal9
In a meta-analysis of 14 studies, LISA, compared with INSURE or delayed ET tube removal, resulted in a significant decrease in:9
- Composite outcome of death or BPD at 36 weeks’ PMA: RD of -0.11 (95% CI: -0.15 to -0.07), NNTB of 9 (95% CI: 7 to 16)
- Need for mechanical ventilation within 72 hours and at any time: RD of -0.14 (95% CI: -0.18 to -0.09), with an NNTB of 8 (95% CI: 6 to 12)
- Cases of intraventricular haemorrhage: RD of -0.04 (95% CI: -0.08 to 0.00), with an NNTB of 22 (95% CI: 12 to 193)
- BPD among survivors at 36 weeks’ PMA: RD of -0.08 (95% CI: -0.11 to -0.04), with an NNTB of 13 (95% CI: 9 to 24)
- Death during the first hospitalisation: RD of -0.02 (95% CI: -0.10 to 0.06), with an NNTB of 20 (95% CI: 12 to 58)
Overall, the meta-analysis indicated that LISA was well tolerated, with similar rates of bradycardia, hypoxaemia, and procedural complications compared with surfactant administration through an ET tube.9
The importance of training and guidelines
Mastering and maintaining the essential skills for surfactant administration can be challenging, especially given the pressures faced by the NHS; however, fast and effective intervention is crucial for optimal RDS care, to get it right first time. 6,12,14,15
Published recommendations from the NHS and BAPM stress that neonatal team members should be trained to agreed competency standards to ensure the successful execution of LISA.12,16 A survey of European NICUs found that a lack of confidence is a major barrier to using the LISA technique, and most centres believe dedicated training would encourage its adoption.17 Improving education and training is one of the key recommendations of the national quality improvement programme, Getting It Right First Time (GIRFT), which also advocates for guidelines to optimise respiratory care and minimise the use of mechanical ventilation.18
Chiesi has developed a range of educational initiatives and workshops to help combat neonatal skill gaps in the UK, which are driven by inadequate training opportunities, workforce shortages, and systemic inefficiencies in resource allocation.19,20
Neonatology Education,
Skills and Training
Chiesi is committed to advancing medical education with innovative and comprehensive training programmes, aiming to equip HCPs with the essential knowledge, skills, and expertise to thrive in the dynamic field of neonatology.
In investing in your professional growth and staying at the forefront of a rapidly evolving medical landscape
Abbreviations
BAPM, British Association of Perinatal Medicine; BPD, bronchopulmonary dysplasia; CPAP, continuous positive airway pressure; ECG, European Consensus Guidelines; ET, endotracheal tube; INSURE, intubate surfactant extubate; LISA, less invasive surfactant administration; LIST, less invasive surfactant therapy; MIST, minimally invasive surfactant therapy; NNT, number needed to treat; NNTB, number needed to treat for an additional beneficial outcome; PMA, post-menstrual age; RD, risk difference.
References
- Dani C, et al. Early Extubation and Nasal Continuous Positive Airway Pressure After Surfactant Treatment for Respiratory Distress Syndrome Among Preterm Infants <30 Weeks’ Gestation Pediatrics. 2004;113:e560–e563.
- Verder H, Nasal continuous positive airway pressure and early surfactant therapy for respiratory distress syndrome in newborns of less than 30 weeks’ gestation et al. Pediatrics. 1999;103:1–6.
- Bohlin K, et al. Implementation of surfactant treatment during continuous positive airway pressure J Perinatol. 2007;27:422–427.
- Leone F, et al. Efficacy of INSURE during nasal CPAP in preterm infants with respiratory distress syndrome Minerva Pediatr. 2013;65:187–192.
- Bui A, et al. Comparison of Efficacy and Pharmacoeconomic Outcomes Between Calfactant and Poractant Alfa in Preterm Infants With Respiratory Distress Syndrome. J Pediatr Pharmacol Ther. 2024;29(3):241–247.
- Sweet DG, et al. European Consensus Guidelines on the Management of Respiratory Distress Syndrome: 2022 Update. Neonatology. 2023;120(1):3–23
- Ho JJ, et al. Continuous positive airway pressure (CPAP) for respiratory distress in preterm infants. Cochrane Database Syst Rev 2020;10(10):CD002271.
- Kribs A et al. Ancillary therapies to enhance success of non-invasive modes of respiratory support – Approaches to delivery room use of surfactant and caffeine? Semin Detal Neonatal Med 2016;21(3):212–218.
- Abdel-Latif MF et al. Surfactant therapy via thin catheter in preterm infants with or at risk of respiratory distress syndrome. Cochrane Database Syst Rev 2021;5(5):CD011672.
- Bao WKJ, et al. Effectiveness and safety profile of introducing less invasive surfactant administration in management of respiratory distress syndrome: A retrospective cohort study in a tertiary neonatal unit in Hong Kong. Pediatr Neonatol. 2024;6:S1875-9572(24)00116-5. doi: 10.1016/j.pedneo.2023.12.013. Epub ahead of print. PMID: 38991861.
- Silveira RC, et al. Less invasive surfactant administration versus intubation-surfactant-extubation in the treatment of neonatal respiratory distress syndrome: a systematic review and meta-analyses. J Pediatr (Rio J). 2024;100(1):8–24.
- BAPM: Neonatal airway safety standard (April 2024). Available at https://hubble-live-assets.s3.eu-west-1.amazonaws.com/bapm/file_asset/file/2585/BAPM_Airway_Standards_Full_April24.pdf. Accessed September 2024.
- Göpel W, et al. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): an open-label, randomised, controlled trial. Lancet. 2011;378(9803):1627–34.
- Hulmes E and El-Kafrawy U. Enhancing trainee endotracheal intubation skills on the NICU. Infant. 2019;15(6).
- BMA: An NHS under pressure. Available at https://www.bma.org.uk/advice-and-support/nhs-delivery-and-workforce/pressures/an-nhs-under-pressure. Accessed August 2024.
- NHS: Three-year delivery plan for maternal and neonatal services. Available at https://www.england.nhs.uk/publication/three-year-delivery-plan-for-maternity-and-neonatal-services/. Accessed September 2024.
- Moretti C, et al. A Survey of the Union of European Neonatal and Perinatal Societies on Neonatal Respiratory Care in Neonatal Intensive Care Units. Children 2024;11;158. https://doi.org/10.3390/children11020158.
- Getting it right first time: A better experience for babies, their families and the neonatal workforce in focus in GIRFT national report. Available at https://gettingitrightfirsttime.co.uk/wp-content/uploads/2022/09/neonatology-overview-DRAFT-v1.0.nhse_.pdf. Accessed September 2024.
- NHS England: Long-term workforce plan. Available at https://www.england.nhs.uk/wp-content/uploads/2023/06/nhs-long-term-workforce-plan-v1.2.pdf. Accessed September 2024.
- The King’s Fund. Improving the allocation of health resources in England. Available at https://www.kingsfund.org.uk/insight-and-analysis/reports/improving-allocation-health-resources-england. Accessed September 2024.
IE-CUR-2400043 | December 2024
Adverse event reporting
For the UK: Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellow Card in the Google Play or Apple App Store. Adverse events should also be reported to Chiesi Limited on 0800 0092329 (UK) or PV.UK@Chiesi.com.
For Ireland: Adverse events should be reported to HPRA Pharmacovigilance – www.hpra.ie. Adverse events should also be reported to Chiesi Limited on 1800 817459 (IE) or PV.UK@Chiesi.com.